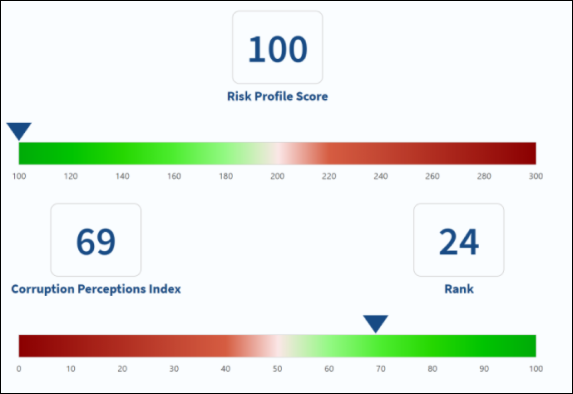
Protect your organization's reputation from third-party risk
Most businesses underestimate their third-party compliance risk until it's too late. Don't be blindsided by unseen bribery or corruption.
Conducting third-party due diligence is an essential component of managing risk and ensuring your organization is protected from fraud. CompliCheck enables you to adopt a risk-based approach to third-party due diligence that is built on guiding principles of global enforcement agencies and regulators.